Answer: The vapor pressure of ethanol at
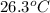 is 238.3 torr.
is 238.3 torr.
Step-by-step explanation:
Given:
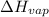 = 38.6 kJ/mol
= 38.6 kJ/mol
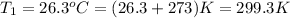
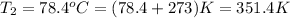
Formula used to calculate the vapor pressure of ethanol is as follows.
![ln(P_(2))/(P_(1)) = (\Delta H_(vap))/(R) [(1)/(T_(1)) - (1)/(T_(2))]\\](https://img.qammunity.org/2022/formulas/chemistry/high-school/4lw3l14yyd1iten8095glllqseou1rhvq0.png)
Substitute the values into above formula as follows.
![ln(P_(2))/(P_(1)) = (\Delta H_(vap))/(R) [(1)/(T_(1)) - (1)/(T_(2))]\\ \\ln (760 torr)/(P_(1)) = (38600 J)/(8.314 J/mol K)[(1)/(299.3) - (1)/(351.4)]\\(760)/(P_(1)) = 3.18\\P_(1) = 238.3 torr](https://img.qammunity.org/2022/formulas/chemistry/high-school/fjj4gte20x2wdd1lsbwu5llnb72iu1tdh7.png)
Thus, we can conclude that the vapor pressure of ethanol at
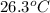 is 238.3 torr.
is 238.3 torr.