Answer: A mass of 0.518 g of phenol must be dissolved in 25.0 g of naphthalene to produce a solution that is 0.22 m in phenol.
Step-by-step explanation:
Given: Mass of naphthalene = 25.0 g
Molality = 0.22 m
This means that 0.22 moles of solute is present per kg of solvent.
As 25.0 g of naphthalene is there that will be 25.0 g per 1000 g (1 kg) is equal to 0.025 kg.
Hence, moles of phenol are calculated as follows.
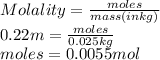
Also, molar mass of phenol is 94.11 g/mol. This means that 1 mole of phenol contains 94.11 g.
Therefore, mass contained by 0.0055 moles of phenol is as follows.
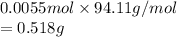
Thus, we can conclude that a mass of 0.518 g of phenol must be dissolved in 25.0 g of naphthalene to produce a solution that is 0.22 m in phenol.