Answer:
A structure that obeys the octet rule for each molecule or ion is
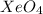
Step-by-step explanation:
Here in
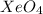 , Xenon has 8 valence electrons and each oxygen atom has 6 valence electrons
, Xenon has 8 valence electrons and each oxygen atom has 6 valence electrons
Lewis structure for
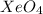 is shown below :
is shown below :
Here , all atoms are having their complete octet .
All the atoms in this Lewis structure is having their complete octet .
Resonance structure is not required as all atoms are same that is oxygen .