Answer:
pH=8.676
Step-by-step explanation:
Given:
0.75 M
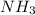
0.20 M
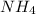
The objective is to calculate the pH of the buffer using the kb for
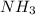
Formula used:
![pOH=pka+log([salt])/([base])\\](https://img.qammunity.org/2022/formulas/chemistry/college/hkopg5vbepi1st8e2zaeo7azoq1mcrkiq2.png)
pH=14-pOH
Solution:
On substituting salt=0.75 and base=0.20 in the formula
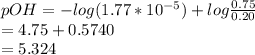
pH=14-pOH
On substituting the pOH value in the above expression,
pH=14-5.324
Therefore,
pH=8.676