Answer: The
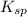 of
of
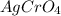 is
is
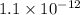 .
.
Step-by-step explanation:
Given:
![[Ag^(+)] = 1.3 * 10^(-4) M](https://img.qammunity.org/2022/formulas/chemistry/high-school/wjpanc8yw5kl2weo1es3cgz2ldo8nbvk1v.png)
The reaction equation will be written as follows.
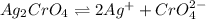
This shows that the concentration of
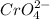 is half the concentration of
is half the concentration of
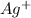 ion. So,
ion. So,
![[CrO^(2-)_(4)] = (1.3 * 10^(-4))/(2)\\= 0.65 * 10^(-4) M](https://img.qammunity.org/2022/formulas/chemistry/high-school/96l61r12udd7mml5uksm8j84mimssu91b5.png)
The expression for
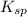 of this reaction is as follows.
of this reaction is as follows.
![K_(sp) = [Ag^(+)]^(2)[CrO^(2-)_(4)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/r1gu6xcyks4b8301cet4p7cu2vcacctl4x.png)
Substitute values into the above expression as follows.
![K_(sp) = [Ag^(+)]^(2)[CrO^(2-)_(4)]\\= (1.3 * 10^(-4))^(2) * 0.65 * 10^(-4)\\= 1.1 * 10^(-12)](https://img.qammunity.org/2022/formulas/chemistry/high-school/wlrqjl8kexniry1vnwq7o55cjyv9lhsbng.png)
Thus, we can conclude that the
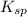 of
of
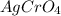 is
is
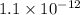 .
.