Answer: Molar mass of the unknown gas is 73.153 g/mol.
Step-by-step explanation:
Given: Mass of each gas = 8.7 g
Volume = 17.28 L
Let us assume that the molar mass of gas is m g/mol.
Molar mass of Ar is 40 g/mol and Ne is 20 g/mol.
Hence, total moles of each gas are as follows.
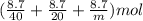
At STP, the total volume of these gases is as follows.
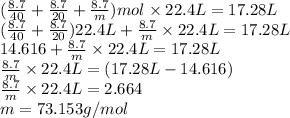
Thus, we can conclude that molar mass of the unknown gas is 73.153 g/mol.