Answer:
Both the technicians are correct.
Step-by-step explanation:
As the pressure increases the temperature will also increase in accordance with the Boyle's law hence greater amount of heat is formed.
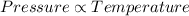
When the temperature increases the intermolecular spaces increase as the molecules of the fuel gain energy and their kinetic energy increases. This is limited to temperatures below dissociation/combustion temperature of diesel .