Answer:
7.8% of the original volume.
Step-by-step explanation:
From the given information:
Temperature
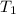 = 22° C = 273 + 22 = 295° C
= 22° C = 273 + 22 = 295° C
Pressure
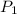 = 240 kPa
= 240 kPa
Temperature
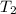 = 45° C
= 45° C
At initial temperature and pressure:
Using the ideal gas equation:
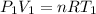
making V_1 (initial volume) the subject:
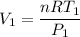
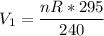
Provided the pressure maintained its rate at 240 kPa, when the temperature reached 45° C, then:
the final volume
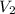 can be computed as:
can be computed as:
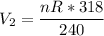
Now, the change in the volume ΔV = V₂ - V₁
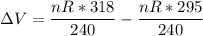
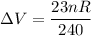
∴
The required fraction of the volume of air to keep up the pressure at (240) kPa can be computed as:
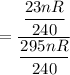
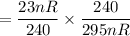
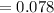
= 7.8% of the original volume.