Answer:
72.96 of water produce by 45.4 L of oxygen at STP.
Step-by-step explanation:
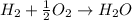
1 mole of oxygen=22.4 L at STP
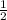 \mole of oxygen=22.4/2=11.2 L
\mole of oxygen=22.4/2=11.2 L
11.2 L of oxygen required to produce water=1 mole
1 L of oxygen required to produce water=1/11.2 mole
45.4 L of oxygen required to produce water=
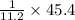
45.4 L of oxygen required to produce water=
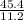 moles
moles
1 mole of water=18 g
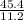 moles of water=
moles of water=
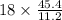
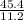 moles of water=72.96 g
moles of water=72.96 g
Hence, 72.96 of water produce by 45.4 L of oxygen at STP.