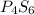Answer:
Compound X has a molar mass of 316.25 g*mol^-1 and the following composition:
element & mass %
phosphorus & 39.18%
sulfur & 60.82%
Write the molecular formula of X.
Step-by-step explanation:
The given molecule of phosphorus and sulfur has molar mass --- 316.25 g.
Empirical formula calculation:
element: phosphorus sulfur
co9mposition: 39.185% 60.82%
divide with
atomic mass: 39.185/31.0 g/mol 60.82/32.0g/mol
=1.26mol 1.90mol
smallest mole ratio: 1.26mol/1.26mol =1 1.90mol/1.26 mol =1.50
multiply with 2: 2 3
Hence, the empirical formula is:
P2S3.
Mass of empirical formula is:
158.0g/mol
Given, molecule has molar mass --- 316.25 g/mol
Hence, the ratio is:
316.25g/mol/158.0 =2
Hence, the molecular formula of the compound is :
2 x (P2S3)
=