Answer:
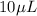 of volume needs to be pipetted out in the test tube.
of volume needs to be pipetted out in the test tube.
Step-by-step explanation:
We are given:
Mass of BSA to be formed =
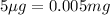 (Conversion factor:
(Conversion factor:
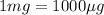
Volume of stock solution =
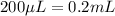 (Conversion factor:
(Conversion factor:
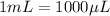
It is also given that for the mass of BSA is 0.5 g, the volume used up is 1 mL
In order to have, 0.005 g, the volume of stock solution needed will be =
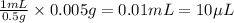
Hence,
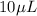 of volume needs to be pipetted out in the test tube.
of volume needs to be pipetted out in the test tube.