Answer:
Specific heat capacity, c = 468.75 J/Kg°C
Step-by-step explanation:
Given the following data;
Power = 1.5 kW to Watts = 1.5 * 1000 = 1500 Watts
Time = 5 seconds
Mass = 0.2 kg
Initial temperature = 20°C
Final temperature = 100°C
To find specific heat capacity;
First of all, we would have to determine the energy consumption of the kettle;
Energy = power * time
Energy = 1500 * 5
Energy = 7500 Joules
Next, we would calculate the specific heat capacity of water.
Heat capacity is given by the formula;
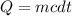
Where;
- Q represents the heat capacity or quantity of heat.
- m represents the mass of an object.
- c represents the specific heat capacity of water.
- dt represents the change in temperature.
dt = T2 - T1
dt = 100 - 20
dt = 80°C
Making c the subject of formula, we have;
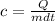
Substituting into the equation, we have;
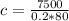
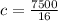
Specific heat capacity, c = 468.75 J/Kg°C