Answer:
21.6 g
Step-by-step explanation:
The reaction that takes place is:
First we convert the given masses of both reactants into moles, using their respective molar masses:
- 9.6 g CH₄ ÷ 16 g/mol = 0.6 mol CH₄
- 64.9 g O₂ ÷ 32 g/mol = 2.03 mol O₂
0.6 moles of CH₄ would react completely with (2 * 0.6) 1.2 moles of O₂. As there are more O₂ moles than required, O₂ is the reactant in excess and CH₄ is the limiting reactant.
Now we calculate how many moles of water are produced, using the number of moles of the limiting reactant:
- 0.6 mol CH₄ *
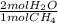 = 1.2 mol H₂O
= 1.2 mol H₂O
Finally we convert 1.2 moles of water into grams, using its molar mass:
- 1.2 mol * 18 g/mol = 21.6 g