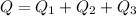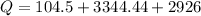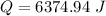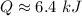Answer:
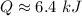
Step-by-step explanation:
Quantity of heat required by 10 gram of ice initially warm it from -5°C to 0°C:
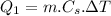
here;
mass, m = 10 g
specific heat capacity of ice,
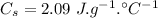
change in temperature,
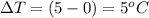
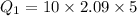
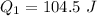
Amount of heat required to melt the ice at 0°C:
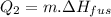
where,
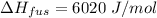
we know that no. of moles is = (wt. in gram)
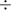 (molecular mass)
(molecular mass)
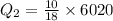
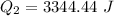
Now, the heat required to bring the water to 70°C from 0°C:
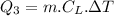
specific heat of water,
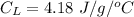
change in temperature,
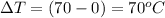
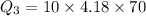
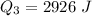
Therefore the total heat required to warm 10.0 grams of ice at -5.0°C to a temperature of 70.0°C: