Answer:
The initial temperature of the water is -75.08 K.
Step-by-step explanation:
Given that,
Mass of water, m = 27 g
Heat absorbed, Q = 1,5000 J
Final temperature of water, T₂ = 57.7 K
The specific heat of water is 4.184 J/g-K
We know that,
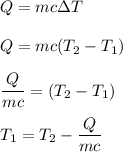
Put all the values,
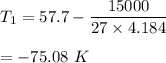
So, the initial temperature of the water is -75.08 K.