Answer: The fractional abundance of lighter isotope is 0.518
Step-by-step explanation:
Average atomic weight is the sum of the masses of the individual isotopes each multiplied by its fractional abundance. The equation used is:
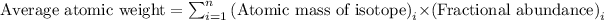 ......(1)
......(1)
Let the fractional abundance of Ag-107 isotope be 'x'
Atomic mass = 106.90509 amu
Fractional abundance = x
Atomic mass = 108.9047 amu
Fractional abundance = (1 - x)
Average atomic mass of silver = 107.8682 amu
Plugging values in equation 1:
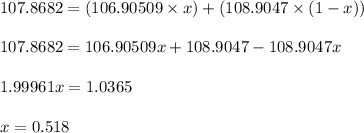
Fractional abundance of Ag-107 isotope (lighter) = x = 0.518
Hence, the fractional abundance of lighter isotope is 0.518