Answer:
Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions: Ca^2 , Al^3 ,I^-,S^2-
Step-by-step explanation:
1)Formula of calcium iodide:
The compound of Ca^2+ and I^-1:
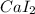
2) Formula of calcium sulfide:
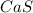
3) Formula of aluminum iodide:
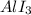
4) Formul aluminum sulfide:
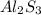
These are the four ionic compounds that are formed.