Answer:
The density of the mixture is 0.775 g/cm³
Step-by-step explanation:
The given parameters are;
The mass of the alcohol, m₁ = 21 g
The density of the alcohol, ρ₁ = 0.7 g/cm³
The mass of the water with which the alcohol is mixed, m₂ = 10 g
The density of wate, ρ₂ = 1.0 g/cm³
The density of a substance is given by the ratio of the mass to the volume of the substance
The density of the mixture, ρ is given as follows;
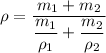
Therefore;
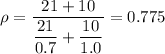
The density of the mixture, ρ = 0.775 g/cm³.