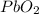Answer:
Find the chemical formulas of oxides that contain the following composition:
S:50%; C:42,8%; Mn:49,6%; Pb:86,6%
Step-by-step explanation:
In the given oxide of sulphur,
50%S is present that means remaining 50% is O.
Divide the % with atomic mass:
S O
50/32= 1.5625 50/16=3.125
divide with smallest ratio:
1.5625/1.5625=1 3.125/1.5625=2
Hence, the formula of oxide is
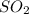 .
.
Similarly:
Carbon is 42.8% means the reamining % will be O that is 57.2%.
C O
42.8/12=3.575 57.2/16=3.575
Divide with smallest ratio:
3.575/3.575=1 3.575/3.575=1
Hence, the formula of oxide is CO.
Mn is 49.6% means the remining is % is O that is 50.4%.
First divide with atmic mass of respective element, then get the smallest ratio.
That gives the mole ratio of each constituent atom.
Mn O
49.6/55.0=0.90 50.4/16=3.15
0.90/0.90=1 3.15/0.90=3.5
Multiply with two
2 7
Hence, the formula becomes:
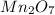
Pb is 86.6% means ---remaining 13.4% is O.
Pb O
86.6/207 = 0.418 mol 13.4/16=0.85
0.418/0.418=1 0.85/0.418=2.0
Hence, the formula is: