Answer: The mass of water produced is 507.6 g
Step-by-step explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
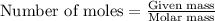 ......(1)
......(1)
Given mass of glucose = 846 g
Molar mass of glucose = 180 g/mol
Plugging values in equation 1:
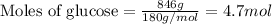
The given chemical equation follows:
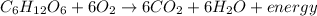
By the stoichiometry of the reaction:
If 1 mole of glucose produces 6 moles of water
So, 4.7 moles of glucose will produce =
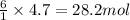 of water
of water
Molar mass of water = 18 g/mol
Plugging values in equation 1:
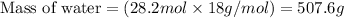
Hence, the mass of water produced is 507.6 g