Answer: The value of
![[H_(3)O^(+)]](https://img.qammunity.org/2022/formulas/chemistry/college/nwtmiafl7iw6che7do1vmidmmgd46r7c92.png) is 0.0012 M and
is 0.0012 M and
![[OH^(-)]](https://img.qammunity.org/2022/formulas/chemistry/college/qjzgvf2re7cxlpp30r8peb2aoxzl0892fu.png) is
is
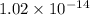 .
.
Step-by-step explanation:
pH is the negative logarithm of concentration of hydrogen ion.
It is given that pH is 2.89. So, the value of concentration of hydrogen ions is calculated as follows.
![pH = - log [H^(+)]\\2.89 = - log [H^(+)]\\conc. H^(+) = 0.0012 M](https://img.qammunity.org/2022/formulas/chemistry/college/lr4pxcdk766b2jl8v3ikvqsmr34efmemf0.png)
The relation between pH and pOH value is as follows.
pH + pOH = 14
0.0012 + pOH = 14
pOH = 14 - 0.0012 = 13.99
Now, pOH is the negative logarithm of concentration of hydroxide ions.
Hence,
![[OH^(-)]](https://img.qammunity.org/2022/formulas/chemistry/college/qjzgvf2re7cxlpp30r8peb2aoxzl0892fu.png) is calculated as follows.
is calculated as follows.
![pOH = - log [OH^(-)]\\13.99 = - log [OH^(-)]\\conc. OH^(-) = 1.02 * 10^(-14) M](https://img.qammunity.org/2022/formulas/chemistry/college/xwfh0x5geji838aih9cuog7v911curhcwk.png)
Thus, we can conclude that the value of
![[H_(3)O^(+)]](https://img.qammunity.org/2022/formulas/chemistry/college/nwtmiafl7iw6che7do1vmidmmgd46r7c92.png) is 0.0012 M and
is 0.0012 M and
![[OH^(-)]](https://img.qammunity.org/2022/formulas/chemistry/college/qjzgvf2re7cxlpp30r8peb2aoxzl0892fu.png) is
is
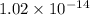 .
.