Answer:
Concentration C = 0.0189 mol/L
Step-by-step explanation:
From the given information:
Let consider the formula used in calculating the concentration according to Beer's law:
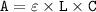 --- (1)
--- (1)
here;
A = absorbance
ε = coefficient of molar absorptivity
L = path length
C = concentration (mol/L)
Also, from Beer law plot:
y = mx+b
where,
y represent absorbance A
b represents intercept
m represents the coefficient of molar absorptivity ε
and x represents the concentration(C).
replacing the substituted entities
A = ε × C + b ---- (2)
Making the concentration the subject of the above formula:
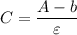 ----(3)
----(3)
From y = 11.589x - 0.0002
A = 11.589 *C - 0.0002
Given that:
A = 0.219
∴
0.219 = 11.589 *C - 0.0002
0.219 + 0.0002 = 11.589 *C
C = 0.2192/11.589
C = 0.0189 mol/L