Answer:
1. Jacques Charles
2. Temperature
3. Volume
4. Pressure
5.
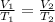
Step-by-step explanation:
Jacques Alexandre-César Charles was a French mathematician, scientist, inventor, balloonist, aeronaut, and physicist who was born on the 12th of November, 1746 in Beaugency, France and died on the 7th of April, 1823 in Paris, France. He was famously known for his invention of the first hydrogen balloon in 1783 and the discovery of Charles Law.
Charles states that when the pressure of an ideal gas is kept constant, the volume of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Charles' law is given by the formula;
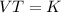
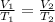
Where;
- V1 and V2 represents the initial and final volumes respectively.
- T1 and T2 represents the initial and final temperatures respectively.