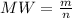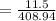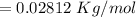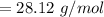Answer:
The molecular weight will be "28.12 g/mol".
Step-by-step explanation:
The given values are:
Pressure,
P = 10 atm
=
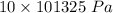
=
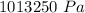
Temperature,
T = 298 K
Mass,
m = 11.5 Kg
Volume,
V = 1000 r
=
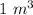
R = 8.3145 J/mol K
Now,
By using the ideal gas law, we get
⇒

o,
⇒
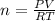
By substituting the values, we get
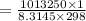
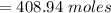
As we know,
⇒
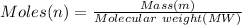
or,
⇒