The complete question is as follows: 2 molecules of potassium chlorate (KClO3) form 2 molecules of potassium chloride (KCl) and 3 molecules of oxygen (O2). Write a balanced equation for this reaction?
Answer: The balanced chemical equation is
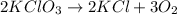 .
.
Step-by-step explanation:
A balanced equation is defined as the equation which contains same number of atoms on both reactant and product side when a chemical reaction occurs.
Hence, when 2 molecules of potassium chlorate
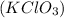 form 2 molecules of potassium chloride (KCl) and 3 molecules of oxygen
form 2 molecules of potassium chloride (KCl) and 3 molecules of oxygen
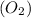 then its balanced equation will be as follows.
then its balanced equation will be as follows.
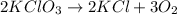
Here, number of atoms on the reactant side as as follows.
Number of atoms on the product side are as follows.
Since, the number of atoms on reactant side are equal to the number of atoms on product side. So, this reaction equation is balanced.
Thus, we can conclude that the balanced chemical equation is
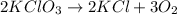 .
.