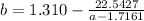Answer:
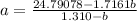
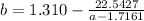
Step-by-step explanation:
Given
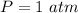
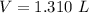
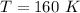
Required
Solve for a and b
Van Der Waals equation is:
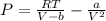
Substitute values for P, V and T, we have:
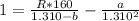
R is a constant and the value is:
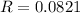
So, the equation becomes:
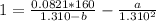
Simplify the expression
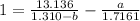 ----- (a)
----- (a)
Solving for (a):
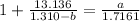
Multiply both sides by 1.7161
![a = [1 + (13.136)/(1.310 - b)] * 1.7161](https://img.qammunity.org/2022/formulas/chemistry/high-school/yzkymvcvb461dlas1aw5pfguumba3n5ewj.png)
Take LCM
![a = [(1.310 - b+13.136)/(1.310 - b)] * 1.7161](https://img.qammunity.org/2022/formulas/chemistry/high-school/iw0kyfmelpmd4eqi8njydqzvosayzqc9ye.png)
Evaluate like terms
![a = [(14.446- b)/(1.310 - b)] * 1.7161](https://img.qammunity.org/2022/formulas/chemistry/high-school/7ia8rzj35l8ktd1t6sihzjkbot109q67ye.png)
Open bracket
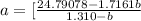
Solving for (b), we have:
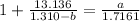
Subtract 1 from both sides
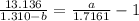
Take LCM
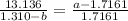
Inverse both sides
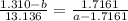
Multiply both sides by 13.136
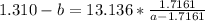
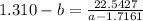
Collect like terms