Answer: -3112 kJ of heat will be released when 5 moles of nitrogen is produced.
Step-by-step explanation:
We are given:
Moles of nitrogen = 5 moles
Given chemical reaction follows:
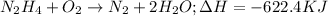
By stoichiometry of the reaction:
If 1 mole of nitrogen gas is produced, the heat released is -622.4 kJ
So, if 5 moles of nitrogen gas is produced, the heat released will be =
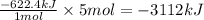
Hence, -3112 kJ of heat will be released when 5 moles of nitrogen is produced.