Answer: The volume of the solution is 85.7 mL
Step-by-step explanation:
Molarity is defined as the amount of solute expressed in the number of moles present per liter of solution. The units of molarity are mol/L. The formula used to calculate molarity:
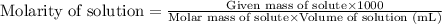 .....(1)
.....(1)
We are given:
Molarity of solution = 0.600 M
Given mass of
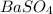 = 12.00 g
= 12.00 g
We know, molar mass of
![BaSO_4=[(1* 137.33)+(1* 32.07)+(4* 16)]=233.4g/mol](https://img.qammunity.org/2022/formulas/chemistry/high-school/ynozm917dx6g4zvk4su5geovkkhfqgz9om.png)
Putting values in equation 1, we get:

The rule of significant number that is applied for the problems having multiplication and division:
The least number of significant figures in any number of the problem determines the number of significant figures in the answer.
Here, the least number of significant figures is 3 that is determined by the number, 0.600. Thus, the answer must have these many significant figures only.
Hence, the volume of the solution is 85.7 mL