Answer:
B) 2.7 g of aluminium has a volume of 1 cm^3
Step-by-step explanation:
Density can be defined as mass all over the volume of an object.
Simply stated, density is mass per unit volume of an object.
Mathematically, density is given by the equation;
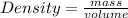
If the density of aluminum is 2.7 g/cm³, it simply means that 2.7 g of aluminium has a volume of 1 cm³
Check:
Given the following data;
Mass = 2.7 grams
Volume = 1 cm³
Substituting into the formula, we have;
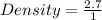
Density = 2.7 g/cm³