Answer: The hydronium
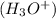 concentration of a solution with a pH of 3.60 is 0.56 M.
concentration of a solution with a pH of 3.60 is 0.56 M.
Step-by-step explanation:
pH of a substance is the negative logarithm of concentration of hydrogen ions present in it.
It's formula is; pH = - log
![[H^(+)]](https://img.qammunity.org/2022/formulas/advanced-placement-ap/high-school/mzxtdst0dvjt3akqb2tr71s7b1u99f0cdo.png)
When pH of a solution is 3.60 then its hydronium or hydrogen ion concentration is calculated as follows.
![pH = - log [H^(+)]\\3.6 = - log [H^(+)]\\concentration of H^(+) = antilog (-3.6)\\= 0.56](https://img.qammunity.org/2022/formulas/chemistry/high-school/a1bueuu8axgk2b5f87u1l8b8ax5ugzvh9n.png)
Thus, we can conclude that the hydronium
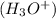 concentration of a solution with a pH of 3.60 is 0.56 M.
concentration of a solution with a pH of 3.60 is 0.56 M.