Answer:
Boyle's law.
Step-by-step explanation:
Robert Boyle was an Irish chemist and is famously referred to as the first modern chemist. He was born on the 25th of January, 1627 in Lismore, Ireland and died on the 31st, December 1691, London, United Kingdom.
Boyles states that when the temperature of an ideal gas is kept constant, the pressure of the gas is inversely proportional to the volume occupied by the gas.
Mathematically, Boyles law is given by;
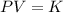
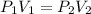
Where;
P1 is the original pressure.
P2 is the final pressure.
V1 is the original volume.
V2 is the final volume.
Hence, when you release some of the paint from a spray paint can by applying an amount of pressure and the can remains at the same temperature, the gas law which this represent is Boyle's law.