Answer:
There is another approach to this problem that
might be more in line with what you are studying ..... NOTE: the first answer is correct.
Explanation:
1 = 2
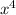
1/2 =
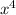
ln(1/2) = 4 ln(x)
ln(1/2)/4 = ln(x)
x =
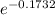
x = .8408
~~~~~~~~~~~~~~~~
model : 840
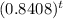
~~~~~~~~~~~~~~~~~
(.03* 840) = 840
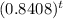
(.25.2) = 840
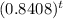
.03 =
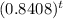
ln(.03 )=ln(
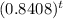 )
)
t = ln(.03 )/ln(0.8408 )
t = 20.22