Answer: 18 moles of
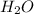 will be produced.
will be produced.
Step-by-step explanation:
We are given:
Moles of ammonia = 12 moles
The given chemical equation follows:
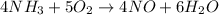
By the stoichiometry of the reaction:
4 moles of ammonia produces 6 moles of water
So, 12 moles of ammonia will produce =
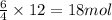 of water
of water
Hence, 18 moles of
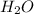 will be produced.
will be produced.