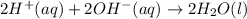Answer: The correct net ionic equation for the reaction is
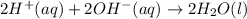
Step-by-step explanation:
Net ionic equation is defined as the equations in which spectator ions are not included.
Spectator ions are the ones that are present equally on the reactant and product sides. They do not participate in the reaction.
The balanced molecular equation is:
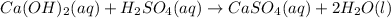
The complete ionic equation follows:
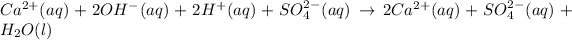
As calcium and sulfate ions are present on both sides of the reaction. Thus, they are considered spectator ions.
The net ionic equation follows:
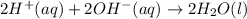
Hence, the correct net ionic equation for the reaction is