Answer: 54 molecules of water will be formed in the reaction.
Step-by-step explanation:
A balanced chemical equation is one where all the individual atoms are equal on both sides of the reaction. It follows the law of conservation of mass.
For the given unbalanced chemical equation, the balanced equation follows:
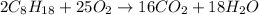
We are given:
Molecules of
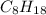 = 6
= 6
Molecules of
 = 75
= 75
By the stoichiometry of the reaction:
If 2 molecules of
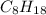 produces 18 molecules of water
produces 18 molecules of water
So, 6 molecules of
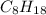 will produce =
will produce =
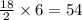 molecules of water
molecules of water
Hence, 54 molecules of water will be formed in the reaction.