Answer: It is true that Your friend should release the
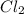 first.
first.
Step-by-step explanation:
The velocity of particles of a gas is inversely proportional to the mass of gas. This means that more is the mass of gas less will be its velocity.
Or, more will be the mass of gas more slowly it will move from one place to another.
The molar mass of chlorine gas is more than the molar mass of hydrogen gas. Therefore, chlorine gas will move slowly.
So, your friend should release the
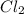 gas first and then according to the length of room you should release the
gas first and then according to the length of room you should release the
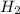 gas.
gas.
Thus, we can conclude that it is true that Your friend should release the
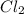 first.
first.