Answer:
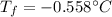
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out possible for us solve this problem by using the equation for the calculation of the freezing point depression as shown below:
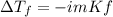
Whereas the the van't Hoff factor of K2SO4 is 3, as it ionizes into two potassium ions and one sulfate ion, the molality is 0.100 m and the freezing point depression constant of water which is 1.86°C/m:
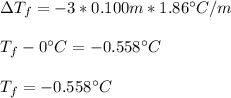
Regards!