Answer: The local atmospheric pressure in that location is 86.1 kPa.
Step-by-step explanation:
Using the rate of evaporation and rate of heat transfer the heat of evaporation is calculated as follows.
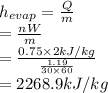
Now, using enthalpy of vaporization the local atmospheric pressure is determined from data in A-5 using interpolation:
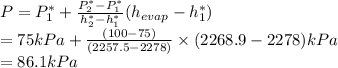
Thus, we can conclude that the local atmospheric pressure in that location is 86.1 kPa.