Answer: The vapor pressure of water in the resulting solution is 20.4 torr
Step-by-step explanation:
Mole fraction is defined as the moles of a component present in the total moles of a solution. It is given by the equation:
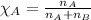 .....(1)
.....(1)
where n is the number of moles
We are given:
Moles of a non-volatile non-electrolyte = 0.600 moles
Moles of water = 3.60 moles
Putting values in equation 1, we get:
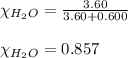
Equation used to calculate the vapor pressure of substance A in a solution is given by:
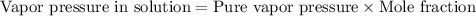
We are given:
Vapor pressure of pure water = 23.8 torr
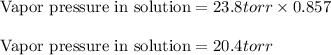
Hence, the vapor pressure of water in the resulting solution is 20.4 torr