Answer: The mass of P-32 left from the original sample is 32.07 mg
Step-by-step explanation:
All radioactive decay processes follow first-order reactions.
Calculating rate constant for first order reaction using half life:
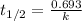 .....(1)
.....(1)
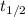 = half life period = 14.3 days
= half life period = 14.3 days
k = rate constant = ?
Putting values in equation 1:
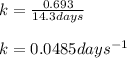
The integrated rate law equation for first-order kinetics:
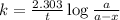 ......(2)
......(2)
Given values:
a = initial concentration of reactant = 175 mg
a - x = concentration of reactant left after time 't' = ? mg
t = time period = 35.0 days
Putting values in equation 2:
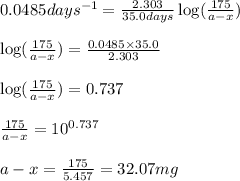
Hence, the mass of P-32 left from the original sample is 32.07 mg