Answer:
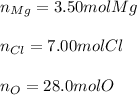
Step-by-step explanation:
Hello there!
In this case, according to the given information it turns out possible for us to realize that one mole of the given compound, Mg(ClO₄)₂, has one mole of Mg, two moles of Cl and eight moles of O; thus, we proceed as follows:
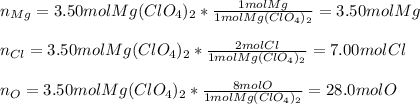
Best regards!