Answer:
W = -262 J.
Step-by-step explanation:
Hello there!
In this case, according to the given information, we can recall the definition of work in terms of constant pressure and variable volume as follows:
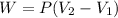
So we plug in the given pressure and volumes to obtain:
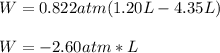
Now, we convert this number to J (Pa*m³) by using the shown below conversion factor:
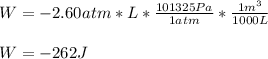
Regards!