Answer: Rank in order the strongest to the weakest acid is
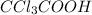 >
>
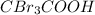 >
>
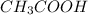 .
.
Step-by-step explanation:
More readily a substance is able to donate a hydrogen ion more will be its acidic strength. Hence, stronger will be the acid.
More is the electronegativity of atoms attached to the acid more easily it will donate a proton. Hence, more will be its acidic strength.
Chlorine is more electronegative in nature as compared to bromine. So,
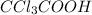 is more acidic than
is more acidic than
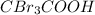 .
.
Since there is no electronegative group attached to
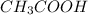 so it is least acidic than
so it is least acidic than
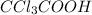 and
and
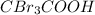 .
.
Thus, we can conclude that rank in order the strongest to the weakest acid is
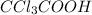 >
>
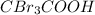 >
>
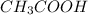 .
.