Answer:
V = 5 m³
Step-by-step explanation:
The density of air, d = 1.2 kg/m³
Mass of the dry air, m = 6 kg
We need to find the volume o the gas. We know that, the density of an object is given by mass divided by its volume. So,
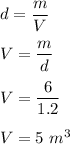
So, the volume of the dry air is 5 m³.