Answer: The final equation has hydroxide ions which indicate that the reaction has occurred in a basic medium.
Step-by-step explanation:
Redox reaction is defined as the reaction in which oxidation and reduction take place simultaneously.
The oxidation reaction is defined as the reaction in which a chemical species loses electrons in a chemical reaction. It occurs when the oxidation number of a species increases.
A reduction reaction is defined as the reaction in which a chemical species gains electrons in a chemical reaction. It occurs when the oxidation number of a species decreases.
The given redox reaction follows:
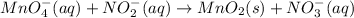
To balance the given redox reaction in basic medium, there are few steps to be followed:
- Writing the given oxidation and reduction half-reactions for the given equation with the correct number of electrons
Oxidation half-reaction:
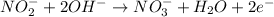
Reduction half-reaction:
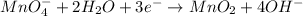
- Multiply each half-reaction by the correct number in order to balance charges for the two half-reactions
Oxidation half-reaction:
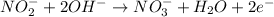 ( × 3)
( × 3)
Reduction half-reaction:
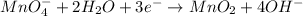 ( × 2)
( × 2)
The half-reactions now become:
Oxidation half-reaction:
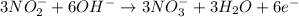
Reduction half-reaction:
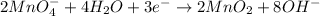
- Add the equations and simplify to get a balanced equation
Overall redox reaction:
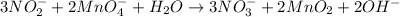
As we can see that in the overall redox reaction, hydroxide ions are released in the solution. Thus, making it a basic solution