The question is incomplete, the complete question is:
A solution of permanganate is standardized by titration with oxalic acid. To react completely with 0.0018 mol of oxalic acid required 28.18 mL of permanganate solution. The unbalanced chemical equation for the reaction in acidic solution is:
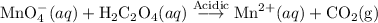
Determine the concentration of the permanganate solution in molarity.
Answer: The molarity of permanganate solution is 0.026 M
Step-by-step explanation:
The balanced chemical equation follows:
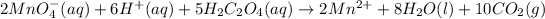
Given values:
Moles of oxalic acid = 0.0018 moles
By the stoichiometry of the reaction:
If 5 moles of oxalic acid reacts with 2 moles of permanganate solution
So, 0.0018 moles of oxalic acid will react with =
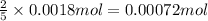 of permanganate solution
of permanganate solution
Molarity is defined as the amount of solute expressed in the number of moles present per liter of solution. The units of molarity are mol/L. The formula used to calculate molarity:
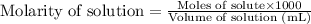 .....(1)
.....(1)
Given values:
Moles of permanganate solution = 0.00072 moles
Volume of solution = 28.18 mL
Putting values in equation 1, we get:
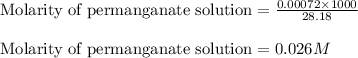
Hence, the molarity of permanganate solution is 0.026 M