Answer:
(a) The moles of CuSO₄ is 3.125 × 10⁻³ moles.
(b) The moles of Cu is 3.125 × 10⁻³ moles.
(c) The mass of Cu is 0.2 g.
Step-by-step explanation:
Given:
Mass of CuSO₄ = 0.5 g
Molar mass of CuSO₄ = 160 g/mol
The given balanced chemical equation is:
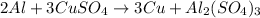
Part (a):
Calculating the moles of CuSO₄.
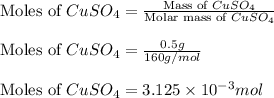
Thus, the moles of CuSO₄ is 3.125 × 10⁻³ moles.
Part (b):
Calculating the moles of Cu.
From the balanced chemical equation, we conclude that:
As, 3 moles of CuSO₄ reacts to give 3 moles of Cu
So, 3.125 × 10⁻³ moles of CuSO₄ reacts to give 3.125 × 10⁻³ moles of Cu
Thus, the moles of Cu is 3.125 × 10⁻³ moles.
Part (c):
Calculating the mass of Cu.
Mass of Cu = Moles of Cu × Molar mass of Cu
Molar mass of Cu = 64 g/mol
Mass of Cu = (3.125 × 10⁻³ mole) × (64 g/mol)
Mass of Cu = 0.2 g
Thus, the mass of Cu is 0.2 g.