Answer:
The number of moles in 89 grams of KCl is approximately 1.193 moles
Step-by-step explanation:
A mole of an element or compound, is the quantity of the element or compound that contains 6.022 × 10²³ molecules of the element or compound, such that the molar mass of one mole is dependent on the mass of each molecule of the element or compound that make up the 6.022 × 10²³ particles
The given parameters are;
The mass of the KCl = 89 grams
The Molar mass, MW, of potassium, K = 39.1 g/mole
The Molar mass, MW, of chlorine, Cl = 35.5 g/mole
Therefore, given that KCl, has one K and one Cl, per mole, we have;
The Molar mass, MW, of potassium chloride, KCl = (39.1 + 35.5) g/mole = 74.6 g/mole
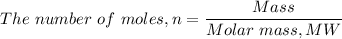
Therefore, the number of moles of KCl in 89 g, 'n' is given as follows;
n = (89 g)/(74.6 g/mole) ≈ 1.193 moles
The number of moles in 89 grams of KCl, n ≈ 1.193 moles.