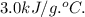Answer:
A 1.0 g sample of propane, C3H8, was burned in the calorimeter.
The temperature rose from 28.5 0C to 32.0 0C and the heat of combustion 10.5 kJ/g.
Calculate the heat capacity of the calorimeter apparatus in kJ/0C
Step-by-step explanation:
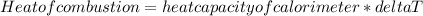
Given,
The heat of combustion = 10.5kJ/g.
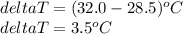
Substitute these values in the above formula to get the value of heat capacity of the calorimeter.
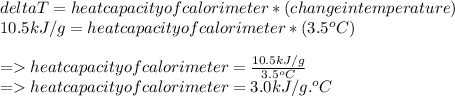
Answer:
The heat capacity of the calorimeter is