Answer:
The efficiency of Carnot's heat engine is 26.8 %.
Step-by-step explanation:
Temperature of hot reservoir, TH = 100 degree C = 373 K
temperature of cold reservoir, Tc = 0 degree C = 273 K
The efficiency of Carnot's heat engine is
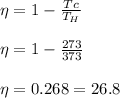
The efficiency of Carnot's heat engine is 26.8 %.